Acid-Base Balance: Respiratory Control and Renal Handling - Explained Clearly
Respiratory Control and Renal Handling of Acid-Base Balance - Explained in 6 min - It's mind-boggling how easy it is!
This lesson includes an animated video lecture, downloadable images, quiz questions and a PDF
pH is an indicator of acidity. The body’s blood pH is strictly regulated within a narrow range between 7.35 and 7.45. This is because even a minor change in acidity may have devastating effects on protein stability and biochemical processes.

Normal cellular metabolism constantly produces and excretes carbon dioxide into the blood. Carbon dioxide combines with water to make carbonic acid which dissociates into hydrogen ions and bicarbonate. 
This is an equilibrium, meaning all the components of the left and right sides co-exist at all times, and the concentration of any component is determined by that of others at any given moment.
The rule of thumb is: an increase in concentration of any component on one side will shift the equation to the other side, leading to increased concentrations of all components on that side, and vice versa.
This equilibrium is central to understand acid-base regulation. Continued carbon dioxide production by all cells of the body drives the equilibrium to the right to generate more hydrogen ions.
Because pH is basically a function of hydrogen ion concentration, more hydrogen means higher acidity and lower pH.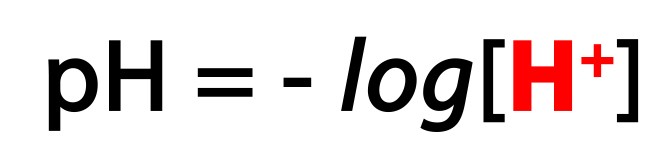
Normal metabolism, therefore, constantly makes the blood more acidic. The body must react to keep the blood pH within the normal limits.
This is achieved by 2 mechanisms:
Subscribe to one of the courses below to continue!
This content is available within the following courses:

Anatomy and Physiology: More than 80 animations, plus downloadable PDFs, downloadable images, and quizzes.

Anatomy and Physiology: More than 80 animations, plus downloadable PDFs, downloadable images, and quizzes.